
Several treatment challenges exist in the management of Ph+ ALL1-5
Explore considerations for adults newly diagnosed with Ph+ ALL
Challenges exist when treating adult patients newly diagnosed with Ph+ ALL1-5
Lack of consensus in frontline treatment
TKIs are part of guideline-recommended treatment regimens1 ; however, none had been FDA-approved.
Aggressive disease with high mutation burden
• Patients with Ph+ ALL have a high probability of developing mutations in BCR::ABL12,3
• Mutations lead to resistance and are a primary cause of relapse after 1st- and 2nd-generation TKIs4
Failure to achieve a complete molecular response
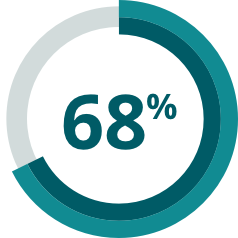
- In a meta-analysis, over 2/3 of patients failed to achieve a complete molecular response with 1st- or 2nd-generation TKIs + chemotherapy5*
- Outcomes with 1st- and 2nd-generation TKIs vary by choice of agent and chemotherapy regimen3
Redefining treatment in
1st-line Ph+ ALL
Comparable safety profile to imatinib in a clinical trial
ANC=absolute neutrophil count; cDNA=complementary DNA; CR=complete remission; mcL=microliter; MRD=minimal residual disease; NCCN=National Comprehensive Cancer Network; Ph+ ALL=Philadelphia chromosome-positive acute lymphoblastic leukemia; TKI=tyrosine kinase inhibitor.